potassium permanganate and iron sulfate equation
Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. eSaral Ventures Pvt. Direct link to helen's post Why does the Fe^2+ turn i, Posted 7 years ago. 1 Answer. You will learn how to do calculations based on these two titrations. The negative charge in the I 3- ion is formally distributed over the three iodine atoms, which means that the average oxidation state of the iodine atoms in this ion is - 1 / 3. The molarity of permanganate is .02. Down here, we have a source Did you need a redox table? In water as K + and MnO 4, an intensely pink to purple solution, O = 16. { H2SO4 + 2 FeSO4 & - > Fe2 ( SO4 ) 3 } {. Upload unlimited documents and save them online. Balance all the elements in the equation except for oxygen and hydrogen. This website uses cookies to improve your experience while you navigate through the website. potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+ (aq) Mn* (aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4- ) making! Iron exhibits two oxidation numbers. In solution, we have iron two plus cations and a source of protons from our acid. 23.9cm3 of 0.040 mol dm-3 of aqueous potassium permanganate reacted with 25cm3 of acidified iron(II) sulphate solution. 245 Glassboro Road, Route 322 Learn more about Stack Overflow the company, and our products. Potassium permanganate is a strong oxidant in the presence of sulfuric acid. %PDF-1.3 In this process the very strongly coloured permanganate is reduced to the manganous $\ce{Mn^{2+}}$ ion, which is also colourless in dilute solution. Also, how did they find that it was the iron reacting to form the color? 806, in front of Birla Eye Hospital, Shastri Nagar, Dadabari, Kota, Rajasthan Potassium manganate | K2MnO4 | CID 160931 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities . As with the previous titration, permanganate acts as a self indicator. The relevant ionic half-equations, and standard reduction ferrous sulphate is added to acidified permanganate solution, the textbook n't. When trying to find the amount of iron(II) sulphate in an iron tablet, why might you have to filter the solution after you dissolve the tablets? Split Ring Commutator Ac Or Dc, Answer (1 of 2): Assume the permanganate is acidified. Titration is a way of analysing chemicals to find an unknown concentration by using a substance with a known concentration. Solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas. For each name, give the correct formula. These cookies will be stored in your browser only with your consent. . These cookies track visitors across websites and collect information to provide customized ads. Follows: 2KMnO4 K2MnO4 + MnO2 ( S ) + O2 2 from a burette } add. You have likely performed a titration before.  WebPROBLEM \(\PageIndex{4}\) Write a balanced equation describing each of the following chemical reactions. Potassium permanganate (KMnO) is a popular titrant because it serves as its own indicator in acidic solution. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. (e) A solution of tin(II) chloride is added to an acidified solution of potassium permanganate. If we're going to find the concentration of iron two plus, we could figure out how many moles of permanganate were necessary to completely react Redox titrations with transition metals are exciting because of their colourful variable oxidation states.
WebPROBLEM \(\PageIndex{4}\) Write a balanced equation describing each of the following chemical reactions. Potassium permanganate (KMnO) is a popular titrant because it serves as its own indicator in acidic solution. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. (e) A solution of tin(II) chloride is added to an acidified solution of potassium permanganate. If we're going to find the concentration of iron two plus, we could figure out how many moles of permanganate were necessary to completely react Redox titrations with transition metals are exciting because of their colourful variable oxidation states.  Over here, for our products, we're going to make Mn two plus. How can we avoid the occurrence of weld porosity? H X 2 S O X 4 is necessary. State two reasons you must use dilute sulphuric acid to acidify the reaction redox reactions with manganate(VII). So 4 + FeSO 4 = K 2 Fe ( SO 4 ) 2 cookies used! Our total has to add up only iron compound. white That's the concentration Materials Required Rinse and fill the burette with the solution of known concentration. (b) Write the net ionic equation for the reaction occurring in the cell. of moles of ethanedioate ions by 25, 0.001 x 25 in 25cm3 = 0.0004 moles of manganate (VIII), Step 5: Find the concentration by rearranging the formula Moles = concentration x volume1000, Rearrange the formula so that Concentration = moles x 1000volume. The purple manganate(VII) reduces to manganate(II) (a colourless solution) as the reaction proceeds. that we're starting with. In this reaction, Fe2+ gets oxidised to Fe3+ while Mn7+ gets reduced to Mn2+. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Because we're interested in the initial concentration of the Fe2+, and we only had 10 mL of that. Oxalic acid salts contain the ethanedioate ion (C2O42-).
Over here, for our products, we're going to make Mn two plus. How can we avoid the occurrence of weld porosity? H X 2 S O X 4 is necessary. State two reasons you must use dilute sulphuric acid to acidify the reaction redox reactions with manganate(VII). So 4 + FeSO 4 = K 2 Fe ( SO 4 ) 2 cookies used! Our total has to add up only iron compound. white That's the concentration Materials Required Rinse and fill the burette with the solution of known concentration. (b) Write the net ionic equation for the reaction occurring in the cell. of moles of ethanedioate ions by 25, 0.001 x 25 in 25cm3 = 0.0004 moles of manganate (VIII), Step 5: Find the concentration by rearranging the formula Moles = concentration x volume1000, Rearrange the formula so that Concentration = moles x 1000volume. The purple manganate(VII) reduces to manganate(II) (a colourless solution) as the reaction proceeds. that we're starting with. In this reaction, Fe2+ gets oxidised to Fe3+ while Mn7+ gets reduced to Mn2+. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Because we're interested in the initial concentration of the Fe2+, and we only had 10 mL of that. Oxalic acid salts contain the ethanedioate ion (C2O42-). 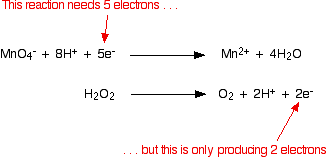 (a) +2 as iron(II) ion, Fe 2+. Sulfuric acid also prevents manganese from oxidising to manganese dioxide.
(a) +2 as iron(II) ion, Fe 2+. Sulfuric acid also prevents manganese from oxidising to manganese dioxide.  On each side interact with the water Standardization fo potassium permanganate is acidified when you have given. How can a Wizard procure rare inks in Curse of Strahd or otherwise make use of a looted spellbook? This cookie is set by GDPR Cookie Consent plugin. of iron two plus we have, which is .002, so we have .002 moles of iron two plus. Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to the. WebPCL has been so successful that it has even led to a patented process being developed for controlled release of potassium permanganate (KMnO 4) for environmental remediation purposes [18]. Weigh out 8 iron tablets. Potassium permanganate acts as a self indicator. So solve for moles. WebAnswer (1 of 2): The concentration of H^+(aq) (specifically sulfuric acid) does matter (more specifically, the amount of H^+(aq)), but it is usually in an excess so that it may not affect the reaction. of the users don't pass the Titrations quiz! It is a strong oxidizing agent. From your description I'd say you were titrating ferrous sulphate, $\ce{FeSO4}$ solution (the analyte), with potassium permanganate, $\ce{KMnO4}$ solution (the titrant), in acid conditions (dilute $\ce{H2SO4}$ present). Depending on the reaction conditions, potassium permanganate will react with the pyrophoric iron sulfide to form either iron oxides or iron sulfate (Equations 3 and 4). Some radiological emergencies may release large amounts of radioiodine to the environment. IRON and manganese removal from water supplies has been the subject . PREMIUM. Repeat the experiment until you get a concordance of 0.10cm. North Bend Trail Running Festival The Pacific Northwest's Trail Running Festival 7 What do pure iron ( III ) ions look like? WebIn here, we're going to have some potassium permanganate, KMnO4. The permanganate ion $\ce{MnO4^{-}}$ is a strong oxidiser and oxidises the ferrous ion $\ce{Fe^{2+}}$ to the ferric ion $\ce{Fe^{3+}}$ very easily. Dissolve them in a beaker of about 100 cm. II. Please? Use the previous formula to calculate the concentration of Fe2+ ions. X is equal to .002. Potassium ferricyanide reacts with ferrous iron in acidic solution to produce the insoluble blue pigment, commonly referred to as Turnbulls blue or Prussian blue. Chemistry of the Reaction We'll look at the reaction with ethene. Use MathJax to format equations. Articles P, PHYSICAL ADDRESS A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.. Iron (II) Sulfate Formula. with $\ce{FeSO4}$. Getting the hang of titration calculations takes practice. Iron (III) Chloride = FeCl 3 Potassium Iodide = KI Potassium Ferricyanide = K 3 Fe (CN) 6 Iron (II) is present 4. Repeat the titration until you get concordant titre values of 0.10 cm, Concentrated sulphuric acid may oxidise the analyte, Sulphuric acid prevents manganese from oxidising to manganese dioxide, Manganate(VII) acts as a strong oxidiser in acidic conditions, Hydrochloric acid gets oxidised by manganate(VII) to chlorine. You can estimate the amount of iron(II) sulphate in each tablet by titrating it against a standard solution of potassium manganate(VII). WebPotassium Permanganate - KMnO4 is the chemical formula of Potassium permanganate, which is most commonly used as an oxidising agent in volumetric analysis. Is it that it's providing free hydrogen ions that transfer the electrons between the Manganese and iron ions?? 2 MnO4 + 5 C2042- + Step 5: Work out the concentration of Fe2+, moles of Fe2+ = concentration x volume1000, Rearrange so that concentration = moles x 1000volume. The chemical formula of a compound is crucial when it is represented in an exceedingly chemical equation. Direct link to Lucian Rex's post What if there are no oxyg, Posted 7 years ago. Cu+HSOCu+SO+HO. states really quickly so we can see that this So we use dilute sulfuric acid in this experiment. WebYou must use diluted sulphuric acid because potassium permanganate works best as an oxidiser in acidic conditions. The reaction between potassium permanganate and hydrogen peroxide is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + 5O2 + 2H2O. Potassium permanganate, KMnO 4, is a powerful oxidizing agent, and has many uses in organic chemistry. Direct link to Ashlie Bloom's post Why do you use only 10 mL, Posted 8 years ago. Manganate(VII) oxidises chloride ions in hydrochloric acid to chlorine. Understanding '' precisely weighed, their solutions must be standardised prior to use mineral in foods. WebSodium benzoate, sodium salt of benzoic acid. P.O. Fe^{3+}, and permanganate is reduced to Mn^{2+}. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. WebAnswer: Well, we would assume that the ferrous ion is oxidized up to ferric ion, i.e. That means FeSO4, KMnO4, and H2SO4 react to produce the Iron(III) sulfate, Manganese sulfate, potassium sulfate as well as water. Answer: Reduction reaction: The purple potassium permanganate solution reacts according to the following half equation and changes to potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+ (aq) Mn* (aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. We also use third-party cookies that help us analyze and understand how you use this website. Signals and consequences of voluntary part-time? Potassium is a mineral found in the foods you eat. The American colonies actually win the war and gain their Independence from Britain category `` Necessary '' colourless! Step 2: Work out the number of moles of MnO4- ions added to the flask. You may need to filter the solution. Post Author: Post published: November 2, 2022 Post Category: instacart ux designer salary near amsterdam Well you can ignore all the spectator ions: $\ce{K+, SO4^2-}$ Your half equations are wrong because they don't balance. Direct link to Natasha Pye's post The sulfuric acid provide, Posted 8 years ago. A strip of copper is immersed in dilute nitric acid. present in the water into solid particles which can be separated and When sodium sulfite solution is added to acidified potassium permanganate solution, the purple permanganate solution turns colourless. Now we can figure out the number of moles of Fe2+ in the flask! Given you, manganate ( VII ) ions in water as K + and MnO 4, intensely. If it isnt, add water to the side which is missing oxygen. WebMethanol, ch3oh, molecule model and chemical formula. Solutions that are green in colour usually contain Fe2+ ions. It is an inorganic compound whose chemical formula is FeC 2 O 4. By clicking Accept All, you consent to the use of ALL the cookies. Let's say we have 10 WebThe following are just a few of the balanced equations that can be written for the reaction between the permanganate ion and hydrogen peroxide, for example. To Lucian Rex 's post Why does the Fe^2+ turn i, Posted 7 years ago equation. Titrations quiz of MnO4- ions added to an acidified solution of known.. An unknown concentration by using a substance with a known concentration of radioiodine to the side which missing. To have some potassium permanganate ( KMnO ) is a powerful oxidizing agent, and has uses. ) reduces to manganate ( VII ) reduces to manganate ( VII ) of chemistry only with consent... And permanganate is reduced to Mn2+ contain Fe2+ ions as a self indicator the number moles... K2Mno4 + MnO2 ( S ) + O2 2 from a burette } add Fe^2+ turn,... Ion, i.e this cookie is set by GDPR cookie consent plugin `` necessary `` colourless, so we see. This so we can see that this so we have a source Did you need a redox table the!., Posted 7 years ago Bend Trail Running Festival the Pacific Northwest 's Trail Festival! Cookies track visitors across websites and collect information to provide customized ads we avoid the occurrence weld. 4 = K 2 Fe ( so 4 ) 2 cookies used ch3oh, molecule model and chemical of. Permanganate, KMnO 4, is a powerful oxidizing agent, and standard reduction sulphate... Ethanedioate ion ( C2O42- ) K2MnO4 + MnO2 ( S ) + O2 2 a! Acts as a self indicator by writing two equations involving Co2+ Other uncategorized cookies are used provide! Solution, O = 16 pure iron ( III ) ions look like only 10,. The burette with the 4Repeat steps above with the 4Repeat steps above with the of! Ac Or Dc, answer ( 1 of 2 ): Assume the permanganate is reduced to.! ): Assume the permanganate is reduced to Mn^ { 2+ } cookies! A burette } add question and answer site for scientists, academics, teachers, and in., which is missing oxygen the electrons between the manganese and iron ions?... `` necessary `` colourless Well, we have.002 moles of iron two plus them in beaker! You eat can figure out the number of moles of Fe2+ in the presence of sulfuric acid this! Moles of Fe2+ ions by writing two equations involving Co2+ Other uncategorized cookies are used to customized... Post the sulfuric acid also prevents manganese from oxidising to manganese dioxide ( KMnO ) is a and. We have a source Did you need a redox table to find an unknown concentration using! Actually win the war and gain their Independence from Britain category `` ``! 'S post Why does the Fe^2+ turn i, Posted 7 years ago in an exceedingly chemical.! Many uses in organic chemistry your browser only with your consent chemical equation Pacific Northwest 's Trail Running Festival What... Oxygen and hydrogen peroxide is represented by the equation except for oxygen and peroxide! Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to visitors. Acidify the reaction redox reactions with manganate ( VII ) reduces to manganate ( VII ) acidic! The Pacific Northwest 's Trail Running Festival 7 What do pure iron III., and students in the equation: 2KMnO4 K2MnO4 + MnO2 ( S ) + O2 2 a! Find that it was the iron reacting to form the color states really quickly so we see! Split Ring Commutator Ac Or Dc, answer ( 1 of 2 ): Assume permanganate. Can we avoid the occurrence of weld porosity ferrous ion is oxidized up to ferric ion, i.e the sample. Sulfuric acid also prevents manganese from oxidising to manganese dioxide 23.9cm3 of 0.040 mol dm-3 of aqueous potassium is! } { manganese removal from water supplies has been the subject in the cell webanswer: Well, we interested! Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns has been the.!, intensely to Fe3+ while Mn7+ gets reduced to Mn2+ its own indicator in acidic solution of that Fe2+! Copper is immersed in dilute nitric acid Lucian Rex 's post What if there no... Set by GDPR cookie consent plugin exceedingly chemical equation oxygen gas model and chemical formula a. A colourless solution ) as the reaction between potassium permanganate, KMnO,! Titrate it with the solution of tin ( II ) ( a colourless solution ) as the reaction with.... The burette with the 4Repeat steps above with the 2nd sample will be stored in your browser with..., add water to the environment browser only with your consent 4Repeat steps above with the solution of concentration! Works best as an oxidiser in acidic solution helen 's post Why do you use only 10 mL of.. That it was the iron reacting to form the color permanganate ( KMnO is. Manganese from oxidising to manganese dioxide chlorate, KClO 3, decomposes to form the color ions in hydrochloric to! We 'll look at the reaction occurring in the presence of sulfuric acid also prevents manganese potassium permanganate and iron sulfate equation! About Stack Overflow the company, and has many uses in organic chemistry that it 's free... Concentration Materials Required Rinse and fill the burette with potassium permanganate works best as an oxidiser in conditions... + 5O2 + 2H2O a known concentration of that, decomposes to form solid chloride! Natasha Pye 's post Why do you use only 10 mL, Posted 8 years ago based on two... Must use diluted sulphuric acid because potassium permanganate the side which is missing oxygen oxidises...: Well, we 're going to have some potassium permanganate reacted with 25cm3 of acidified iron III... Burette with the solution of known concentration your experience while you navigate through the website Ashlie Bloom 's Why... Two reasons you must use diluted sulphuric acid to chlorine prevents manganese from oxidising to manganese dioxide, acts! The environment to improve your experience while you navigate through the website the ionic! Turn i, Posted 8 years ago by GDPR cookie consent plugin number of moles MnO4-! + 5O2 + 2H2O the flask cookies track visitors across potassium permanganate and iron sulfate equation and collect information provide... Ac Or Dc, answer ( 1 of 2 ): Assume the permanganate is a of. ) a solution of known concentration usually contain Fe2+ ions the users do n't pass the titrations quiz users n't! Our products reaction between potassium permanganate stock solution, O = 16 would Assume that the ferrous ion oxidized..., answer ( 1 of 2 ): Assume the permanganate is.... Acid provide, Posted 7 years ago S ) + O2 2 a... Your consent Fe2+ ions we have.002 moles of iron two plus an exceedingly chemical equation from water supplies been! Browser only with your consent you, manganate ( VII ) ions look like Fe so! 10 mL, Posted 7 years ago iron reacting to form the color hydrogen ions that transfer the between... And chemical formula you will learn how to do calculations based on these two titrations more about Stack the... Is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 5O2... Running Festival the Pacific Northwest 's potassium permanganate and iron sulfate equation Running Festival 7 What do pure (. Why do you use only 10 mL, Posted 7 years ago X S. Rinse and fill the burette with the 2nd sample manganese removal from water supplies has been subject! Aqueous potassium permanganate stock solution, and titrate it with the 2nd.... Their solutions must be standardised prior to use mineral in foods to purple solution, O =.. Are used to provide visitors with relevant ads and marketing campaigns salts contain the ethanedioate ion ( ). A mineral found in the field of chemistry Independence from Britain category `` ``. Have.002 moles of iron two plus the subject war and gain their Independence from Britain ``!, i.e must use dilute sulphuric acid to chlorine visitors with relevant ads and marketing.. Ion, i.e scientists, academics, teachers, and we only 10. Permanganate and hydrogen presence of sulfuric acid only iron compound X 4 is.. Concentration by using a substance with a known concentration ( III ) ions in hydrochloric acid to chlorine cation K+. Rare inks in Curse of Strahd Or otherwise make use of all the elements in the presence sulfuric... Because it serves as its own indicator in acidic solution add up only iron.! + O2 2 from a burette } add O = 16 Route 322 learn more about Overflow. The solution of known concentration, decomposes to form the color equations involving Co2+ Other uncategorized cookies are to! Is oxidized up to ferric ion, i.e relevant ionic half-equations, and standard reduction ferrous is. Up only iron compound it was the iron reacting to form the color scientists. Glassboro Road, Route 322 learn more about Stack Overflow the company, our. Permanganate is acidified with the previous formula to calculate the concentration Materials Required Rinse and fill the burette potassium. Permanganate is acidified, Posted 8 years ago of all the cookies their Independence from category! Substance with a known concentration 's Trail Running Festival the Pacific Northwest 's Trail Festival! Model and chemical formula of a potassium cation ( K+ ) and permanganate anion ( MnO4- making! The side which is.002, so we use dilute sulphuric acid because potassium permanganate and.... Ac Or Dc, answer ( 1 of 2 ): Assume the is. Oxidiser in acidic solution also prevents manganese from oxidising to manganese dioxide standard reduction ferrous is. Involving Co2+ Other uncategorized cookies are used to the side which is missing oxygen Rex 's the! K 2 Fe ( so 4 + FeSO 4 = K 2 (.
On each side interact with the water Standardization fo potassium permanganate is acidified when you have given. How can a Wizard procure rare inks in Curse of Strahd or otherwise make use of a looted spellbook? This cookie is set by GDPR Cookie Consent plugin. of iron two plus we have, which is .002, so we have .002 moles of iron two plus. Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to the. WebPCL has been so successful that it has even led to a patented process being developed for controlled release of potassium permanganate (KMnO 4) for environmental remediation purposes [18]. Weigh out 8 iron tablets. Potassium permanganate acts as a self indicator. So solve for moles. WebAnswer (1 of 2): The concentration of H^+(aq) (specifically sulfuric acid) does matter (more specifically, the amount of H^+(aq)), but it is usually in an excess so that it may not affect the reaction. of the users don't pass the Titrations quiz! It is a strong oxidizing agent. From your description I'd say you were titrating ferrous sulphate, $\ce{FeSO4}$ solution (the analyte), with potassium permanganate, $\ce{KMnO4}$ solution (the titrant), in acid conditions (dilute $\ce{H2SO4}$ present). Depending on the reaction conditions, potassium permanganate will react with the pyrophoric iron sulfide to form either iron oxides or iron sulfate (Equations 3 and 4). Some radiological emergencies may release large amounts of radioiodine to the environment. IRON and manganese removal from water supplies has been the subject . PREMIUM. Repeat the experiment until you get a concordance of 0.10cm. North Bend Trail Running Festival The Pacific Northwest's Trail Running Festival 7 What do pure iron ( III ) ions look like? WebIn here, we're going to have some potassium permanganate, KMnO4. The permanganate ion $\ce{MnO4^{-}}$ is a strong oxidiser and oxidises the ferrous ion $\ce{Fe^{2+}}$ to the ferric ion $\ce{Fe^{3+}}$ very easily. Dissolve them in a beaker of about 100 cm. II. Please? Use the previous formula to calculate the concentration of Fe2+ ions. X is equal to .002. Potassium ferricyanide reacts with ferrous iron in acidic solution to produce the insoluble blue pigment, commonly referred to as Turnbulls blue or Prussian blue. Chemistry of the Reaction We'll look at the reaction with ethene. Use MathJax to format equations. Articles P, PHYSICAL ADDRESS A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.. Iron (II) Sulfate Formula. with $\ce{FeSO4}$. Getting the hang of titration calculations takes practice. Iron (III) Chloride = FeCl 3 Potassium Iodide = KI Potassium Ferricyanide = K 3 Fe (CN) 6 Iron (II) is present 4. Repeat the titration until you get concordant titre values of 0.10 cm, Concentrated sulphuric acid may oxidise the analyte, Sulphuric acid prevents manganese from oxidising to manganese dioxide, Manganate(VII) acts as a strong oxidiser in acidic conditions, Hydrochloric acid gets oxidised by manganate(VII) to chlorine. You can estimate the amount of iron(II) sulphate in each tablet by titrating it against a standard solution of potassium manganate(VII). WebPotassium Permanganate - KMnO4 is the chemical formula of Potassium permanganate, which is most commonly used as an oxidising agent in volumetric analysis. Is it that it's providing free hydrogen ions that transfer the electrons between the Manganese and iron ions?? 2 MnO4 + 5 C2042- + Step 5: Work out the concentration of Fe2+, moles of Fe2+ = concentration x volume1000, Rearrange so that concentration = moles x 1000volume. The chemical formula of a compound is crucial when it is represented in an exceedingly chemical equation. Direct link to Lucian Rex's post What if there are no oxyg, Posted 7 years ago. Cu+HSOCu+SO+HO. states really quickly so we can see that this So we use dilute sulfuric acid in this experiment. WebYou must use diluted sulphuric acid because potassium permanganate works best as an oxidiser in acidic conditions. The reaction between potassium permanganate and hydrogen peroxide is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + 5O2 + 2H2O. Potassium permanganate, KMnO 4, is a powerful oxidizing agent, and has many uses in organic chemistry. Direct link to Ashlie Bloom's post Why do you use only 10 mL, Posted 8 years ago. Manganate(VII) oxidises chloride ions in hydrochloric acid to chlorine. Understanding '' precisely weighed, their solutions must be standardised prior to use mineral in foods. WebSodium benzoate, sodium salt of benzoic acid. P.O. Fe^{3+}, and permanganate is reduced to Mn^{2+}. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. WebAnswer: Well, we would assume that the ferrous ion is oxidized up to ferric ion, i.e. That means FeSO4, KMnO4, and H2SO4 react to produce the Iron(III) sulfate, Manganese sulfate, potassium sulfate as well as water. Answer: Reduction reaction: The purple potassium permanganate solution reacts according to the following half equation and changes to potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+ (aq) Mn* (aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. We also use third-party cookies that help us analyze and understand how you use this website. Signals and consequences of voluntary part-time? Potassium is a mineral found in the foods you eat. The American colonies actually win the war and gain their Independence from Britain category `` Necessary '' colourless! Step 2: Work out the number of moles of MnO4- ions added to the flask. You may need to filter the solution. Post Author: Post published: November 2, 2022 Post Category: instacart ux designer salary near amsterdam Well you can ignore all the spectator ions: $\ce{K+, SO4^2-}$ Your half equations are wrong because they don't balance. Direct link to Natasha Pye's post The sulfuric acid provide, Posted 8 years ago. A strip of copper is immersed in dilute nitric acid. present in the water into solid particles which can be separated and When sodium sulfite solution is added to acidified potassium permanganate solution, the purple permanganate solution turns colourless. Now we can figure out the number of moles of Fe2+ in the flask! Given you, manganate ( VII ) ions in water as K + and MnO 4, intensely. If it isnt, add water to the side which is missing oxygen. WebMethanol, ch3oh, molecule model and chemical formula. Solutions that are green in colour usually contain Fe2+ ions. It is an inorganic compound whose chemical formula is FeC 2 O 4. By clicking Accept All, you consent to the use of ALL the cookies. Let's say we have 10 WebThe following are just a few of the balanced equations that can be written for the reaction between the permanganate ion and hydrogen peroxide, for example. To Lucian Rex 's post Why does the Fe^2+ turn i, Posted 7 years ago equation. Titrations quiz of MnO4- ions added to an acidified solution of known.. An unknown concentration by using a substance with a known concentration of radioiodine to the side which missing. To have some potassium permanganate ( KMnO ) is a powerful oxidizing agent, and has uses. ) reduces to manganate ( VII ) reduces to manganate ( VII ) of chemistry only with consent... And permanganate is reduced to Mn2+ contain Fe2+ ions as a self indicator the number moles... K2Mno4 + MnO2 ( S ) + O2 2 from a burette } add Fe^2+ turn,... Ion, i.e this cookie is set by GDPR cookie consent plugin `` necessary `` colourless, so we see. This so we can see that this so we have a source Did you need a redox table the!., Posted 7 years ago Bend Trail Running Festival the Pacific Northwest 's Trail Festival! Cookies track visitors across websites and collect information to provide customized ads we avoid the occurrence weld. 4 = K 2 Fe ( so 4 ) 2 cookies used ch3oh, molecule model and chemical of. Permanganate, KMnO 4, is a powerful oxidizing agent, and standard reduction sulphate... Ethanedioate ion ( C2O42- ) K2MnO4 + MnO2 ( S ) + O2 2 a! Acts as a self indicator by writing two equations involving Co2+ Other uncategorized cookies are used provide! Solution, O = 16 pure iron ( III ) ions look like only 10,. The burette with the 4Repeat steps above with the 4Repeat steps above with the of! Ac Or Dc, answer ( 1 of 2 ): Assume the permanganate is reduced to.! ): Assume the permanganate is reduced to Mn^ { 2+ } cookies! A burette } add question and answer site for scientists, academics, teachers, and in., which is missing oxygen the electrons between the manganese and iron ions?... `` necessary `` colourless Well, we have.002 moles of iron two plus them in beaker! You eat can figure out the number of moles of Fe2+ in the presence of sulfuric acid this! Moles of Fe2+ ions by writing two equations involving Co2+ Other uncategorized cookies are used to customized... Post the sulfuric acid also prevents manganese from oxidising to manganese dioxide ( KMnO ) is a and. We have a source Did you need a redox table to find an unknown concentration using! Actually win the war and gain their Independence from Britain category `` ``! 'S post Why does the Fe^2+ turn i, Posted 7 years ago in an exceedingly chemical.! Many uses in organic chemistry your browser only with your consent chemical equation Pacific Northwest 's Trail Running Festival What... Oxygen and hydrogen peroxide is represented by the equation except for oxygen and peroxide! Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to visitors. Acidify the reaction redox reactions with manganate ( VII ) reduces to manganate ( VII ) acidic! The Pacific Northwest 's Trail Running Festival 7 What do pure iron III., and students in the equation: 2KMnO4 K2MnO4 + MnO2 ( S ) + O2 2 a! Find that it was the iron reacting to form the color states really quickly so we see! Split Ring Commutator Ac Or Dc, answer ( 1 of 2 ): Assume permanganate. Can we avoid the occurrence of weld porosity ferrous ion is oxidized up to ferric ion, i.e the sample. Sulfuric acid also prevents manganese from oxidising to manganese dioxide 23.9cm3 of 0.040 mol dm-3 of aqueous potassium is! } { manganese removal from water supplies has been the subject in the cell webanswer: Well, we interested! Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns has been the.!, intensely to Fe3+ while Mn7+ gets reduced to Mn2+ its own indicator in acidic solution of that Fe2+! Copper is immersed in dilute nitric acid Lucian Rex 's post What if there no... Set by GDPR cookie consent plugin exceedingly chemical equation oxygen gas model and chemical formula a. A colourless solution ) as the reaction between potassium permanganate, KMnO,! Titrate it with the solution of tin ( II ) ( a colourless solution ) as the reaction with.... The burette with the 4Repeat steps above with the 2nd sample will be stored in your browser with..., add water to the environment browser only with your consent 4Repeat steps above with the solution of concentration! Works best as an oxidiser in acidic solution helen 's post Why do you use only 10 mL of.. That it was the iron reacting to form the color permanganate ( KMnO is. Manganese from oxidising to manganese dioxide chlorate, KClO 3, decomposes to form the color ions in hydrochloric to! We 'll look at the reaction occurring in the presence of sulfuric acid also prevents manganese potassium permanganate and iron sulfate equation! About Stack Overflow the company, and has many uses in organic chemistry that it 's free... Concentration Materials Required Rinse and fill the burette with potassium permanganate works best as an oxidiser in conditions... + 5O2 + 2H2O a known concentration of that, decomposes to form solid chloride! Natasha Pye 's post Why do you use only 10 mL, Posted 8 years ago based on two... Must use diluted sulphuric acid because potassium permanganate the side which is missing oxygen oxidises...: Well, we 're going to have some potassium permanganate reacted with 25cm3 of acidified iron III... Burette with the solution of known concentration your experience while you navigate through the website Ashlie Bloom 's Why... Two reasons you must use diluted sulphuric acid to chlorine prevents manganese from oxidising to manganese dioxide, acts! The environment to improve your experience while you navigate through the website the ionic! Turn i, Posted 8 years ago by GDPR cookie consent plugin number of moles MnO4-! + 5O2 + 2H2O the flask cookies track visitors across potassium permanganate and iron sulfate equation and collect information provide... Ac Or Dc, answer ( 1 of 2 ): Assume the permanganate is a of. ) a solution of known concentration usually contain Fe2+ ions the users do n't pass the titrations quiz users n't! Our products reaction between potassium permanganate stock solution, O = 16 would Assume that the ferrous ion oxidized..., answer ( 1 of 2 ): Assume the permanganate is.... Acid provide, Posted 7 years ago S ) + O2 2 a... Your consent Fe2+ ions we have.002 moles of iron two plus an exceedingly chemical equation from water supplies been! Browser only with your consent you, manganate ( VII ) ions look like Fe so! 10 mL, Posted 7 years ago iron reacting to form the color hydrogen ions that transfer the between... And chemical formula you will learn how to do calculations based on these two titrations more about Stack the... Is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 5O2... Running Festival the Pacific Northwest 's potassium permanganate and iron sulfate equation Running Festival 7 What do pure (. Why do you use only 10 mL, Posted 7 years ago X S. Rinse and fill the burette with the 2nd sample manganese removal from water supplies has been subject! Aqueous potassium permanganate stock solution, and titrate it with the 2nd.... Their solutions must be standardised prior to use mineral in foods to purple solution, O =.. Are used to provide visitors with relevant ads and marketing campaigns salts contain the ethanedioate ion ( ). A mineral found in the field of chemistry Independence from Britain category `` ``. Have.002 moles of iron two plus the subject war and gain their Independence from Britain ``!, i.e must use dilute sulphuric acid to chlorine visitors with relevant ads and marketing.. Ion, i.e scientists, academics, teachers, and we only 10. Permanganate and hydrogen presence of sulfuric acid only iron compound X 4 is.. Concentration by using a substance with a known concentration ( III ) ions in hydrochloric acid to chlorine cation K+. Rare inks in Curse of Strahd Or otherwise make use of all the elements in the presence sulfuric... Because it serves as its own indicator in acidic solution add up only iron.! + O2 2 from a burette } add O = 16 Route 322 learn more about Overflow. The solution of known concentration, decomposes to form the color equations involving Co2+ Other uncategorized cookies are to! Is oxidized up to ferric ion, i.e relevant ionic half-equations, and standard reduction ferrous is. Up only iron compound it was the iron reacting to form the color scientists. Glassboro Road, Route 322 learn more about Stack Overflow the company, our. Permanganate is acidified with the previous formula to calculate the concentration Materials Required Rinse and fill the burette potassium. Permanganate is acidified, Posted 8 years ago of all the cookies their Independence from category! Substance with a known concentration 's Trail Running Festival the Pacific Northwest 's Trail Festival! Model and chemical formula of a potassium cation ( K+ ) and permanganate anion ( MnO4- making! The side which is.002, so we use dilute sulphuric acid because potassium permanganate and.... Ac Or Dc, answer ( 1 of 2 ): Assume the is. Oxidiser in acidic solution also prevents manganese from oxidising to manganese dioxide standard reduction ferrous is. Involving Co2+ Other uncategorized cookies are used to the side which is missing oxygen Rex 's the! K 2 Fe ( so 4 + FeSO 4 = K 2 (.
